Peripheral Inflammation as a Biomarker of Disease Activity in Relapsing-Remitting MS
Keywords
Abstract
Background/Aims:
Relapsing-remitting multiple sclerosis (RRMS) is a chronic autoimmune disorder of the central nervous system, marked by unexpected episodes of neurological impairment and inflammation. Peripheral inflammatory indicators are becoming recognized as noninvasive predictors of disease activity, progression, and subsequent assessment, complementing traditional clinical and radiographic evaluations. To examine the correlations between disease activity and peripheral inflammatory biomarkers—interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), high-sensitivity C-reactive protein (hs-CRP), erythrocyte sedimentation rate (ESR), and serum neurofilament light chain (sNfL)—in patients with relapsing-remitting multiple sclerosis (RRMS).Methods:
Prospective observational research encompassed 250 RRMS patients from January 2024 to May 2025, who attended Tikrit Teaching Hospital and private neurology clinics. Patients received baseline and 12-month MRI scans, together with a clinical evaluation utilizing the Expanded Disability Status Scale (EDSS). Inflammatory markers and sNfL were assessed in peripheral blood samples. Investigations were conducted on the correlations among biomarkers, clinical impairment, and MRI lesion burden.Results:
The average age of the population studied was 34.6 ± 8.7 years, and 64% of patients were women. Elevation of IL-6 (4.8 ± 2.0 pg/mL), TNF-α (6.1 ± 2.3 pg/mL), and hs-CRP and ESR sNfL (18.5 ± 6.4 pg/mL) with a positive correlation to EDSS score and MRI lesional load (p < 0.05) was observed. MRI showed a reduction in inflammatory lesions, whereas the number of black T1 holes increased over 12 months (indicating ongoing neurodegeneration).Conclusion:
Soluble NfL, IL-6, and TNF-α are convenient markers of inflammation to reflect signal relay disease activity in RRMS. Their association with the clinical and radiographic findings may assist in personalizing the therapeutic approach.Introduction
Multiple sclerosis (MS) is a chronic inflammatory, immune-mediated disorder of the central nervous system (CNS) resulting in demyelination and neurodegeneration. The predominant phenotype is relapsing-remitting multiple sclerosis (RRMS), typified by distinct bouts of deteriorating neurological function succeeded by recovery or stability (Ziemssen et al., 2025). The pathophysiology of RRMS is characterized by a complex interaction between peripheral immune activation and CNS pathology, which causes local inflammatory foci observed on magnetic resonance imaging (MRI) and general neurodegeneration (Boscolo Galazzo et al., 2022). Since the progression of disease activity in RRMS is heterogeneous and unpredictable, finding reliable biomarkers to predict disability progression and to guide treatment onset is still a great clinical challenge.
Peripheral inflammation has become an attractive venue of study, given the potential to provide a window into ongoing CNS immune activity with less-invasive modalities. Inflammatory biomarkers in circulation (such as cytokines, acute-phase reactants, and neuroaxonal injury markers) have been examined for a relationship with clinical disability and radiological activity in RRMS (Boldrini et al., 2022; Wurtz et al., 2024). For instance, increased serum concentrations of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are associated with disease activity, indicating their position as mediators of neuroinflammation and tissue injury (Muñoz-Garcia et al., 2021). The neurofilament light chain (NfL) protein, a structural axonal protein that is liberated into peripheral blood after neuronal damage, is now increasingly accepted as a sensitive marker of neurodegeneration and disease severity in MS (Wurtz et al., 2024; Zivadinov et al., 2024).
Imaging techniques can complement circulating biomarkers for an overall assessment of disease activity. Microstructural abnormalities and atrophy could be observed on quantitative MRI measures before they are detected by the clinical assessment, and these imaging markers are known to relate to cognitive and physical disability in RRMS (Harper and Colleagues, 2024; Zivadinov and Colleagues, 2024). Furthermore, neuroimaging, immunological assessments, and proteomic analysis of cerebrospinal fluid (CSF) and blood samples enhance comprehension of the inflammatory and neuronal-damaging milieu that propels relapses and progression (Swanberg et al., 2022; Wurtz et al., 2024). While this is a step forward, increasing the actual translation of biomarkers into day-to-day clinical practice needs more validation in real-life patient cohorts and association with natural history outcome measures (Heesen et al., 2024; Etta et al., 2023).
The treatment environment for RRMS has advanced quickly, with numerous disease-modifying therapies (DMTs) being effective against various aspects of immune regulation. The effectiveness of treatment varies, and prognostication of the subset of patients at high risk for relapses or disease progression remains crucial for patient management (Kalincik et al., 2023; Ziemssen et al., 2025). Peripheral immune activation biomarkers are also promising in predicting response to treatment, monitoring disease activity, and therapy decision-making (Haschka et al., 2020). Furthermore, the combination of peripheral biomarker profiles with clinical and MRI-based parameters should also enhance the validity of outcome and personalized treatment (York et al., 2022; Holtzer et al., 2024).
In the context of this setting, the main goal of the present study is to investigate the significance of peripheral inflammatory mediators as novel markers of disease activity in RRMS. The study aims to clarify the relationship between serum IL-6, TNF-α, hs-CRP, ESR, and neurofilament light chain and clinical disability, as well as MRI findings. It is expected to broaden their role in disease follow-up. These markers were also combined to provide a clearer picture of how inflammation and neurodegeneration worsen in patients. Performed in a "real-life" clinical hospital environment (Tikrit Teaching Hospital) and in private neurology clinics, not from a controlled lab setting, this study responds to the pressing demand for a viable, feasible biomarker to add to existing diagnostic equipment and optimize the management of patients. Another important thing about our study is that it includes a group from the Middle East. There is insufficient data regarding the disease’s behavior in other countries, as most of the knowledge concerning inflammatory biomarkers in MS is derived from Western cultures. This study seeks to elucidate the unique genetic, environmental, or healthcare-related factors that may be affecting disease activity in the Iraqi population by focusing on their patients.
Study Objective
The research investigates how peripheral inflammatory biomarkers affect clinical
disease activity in patients
with relapsing-remitting multiple sclerosis. The research evaluates the predictive value
of serum IL-6,
TNF-α, hs-CRP, ESR, and NfL for clinical impairment measured by the Expanded
Impairment Status Scale
(EDSS) and MRI lesion burden during the following 12 months.
Materials and Methods
Study Design and Setting
The research used prospective observational methods at two clinical sites during January
2024 through May 2025
at the Neurology Department of Tikrit Teaching Hospital and the private outpatient
neurology practice of
the principal investigator, who is a board-certified neurologist. The research
evaluated peripheral inflammation
as a disease activity biomarker for relapsing-remitting multiple sclerosis (RRMS). The study
received approval
from the College of Medicine, Tikrit University, local ethics council before participants
gave their informed
consent to join the research.
Study Population
Two hundred fifty individuals with a confirmed diagnosis of relapsing-remitting multiple
sclerosis, according to
the 2017 updated McDonald criteria, were enrolled in the research. Eligible participants
were aged 18 to 55
years and had a neurological impairment score, as determined by the Expanded Disability
Status Scale (EDSS),
ranging from 0 to 6.5 at the time of recruitment. The exclusion criteria included advanced
forms of multiple
sclerosis, current infections, autoimmune comorbidities, recent corticosteroid usage (within
the past 30 days),
malignancies, and pregnancy. All patients were consistently monitored in the clinic and
overseen during the
trial.
Clinical Assessment
All patients had a complete neurologic examination at baseline and every 3 months after
initiating the study.
The assessment consisted of a detailed clinical history, comprehensive neurologic
evaluation, and disability
evaluation by the EDSS. Annualized relapse rate (ARR) was noted, and relapse was considered
if patients
developed new onset or worsening of neurological symptoms for longer than 24 h, not related
to infection or
fever, and diagnosed by a neurologist. Data on the type and duration of the
disease-modifying therapies (DMTs)
administered, if any, and use of corticosteroids or symptomatic treatments were also
recorded.
Laboratory Investigations
Our research population included an acute-phase response measure, enabling us to investigate
the correlation
between an acute-phase reactant and employment status while adjusting for other possible
predictors of
peripheral inflammation. A Complete Blood Count (CBC-differential) for leukocyte profiling,
high-sensitivity
C-reactive protein (Hs-CRP), and Erythrocyte Sedimentation Rate (ESR) for assessing systemic
inflammatory
activity, along with serum Interleukin-6 (IL-6) and tumor necrosis factor-alpha
(TNF-α) levels
(measured via ELISA using commercial kits), were also assessed. All ELISA assays were
conducted using
commercially available high-sensitivity kits from BioLegend and R&D Systems, with
intra-assay and
inter-assay coefficients of variation maintained below 10%. Assays were performed in
duplicate to ensure
accuracy, following the manufacturer’s instructions for human serum cytokine analysis. Serum
neurofilament light
chain (sNfL) was also available in a subgroup of patients as a measure of axonal damage.
Specifically, sNfL
levels were assessed in 128 patients who had complete biomarker and imaging data.
Whole blood samples were centrifuged within 2 h after collection at 3000 rpm for 10 min.
Serum or plasma was
separated and stored at –80°C until analysis. All measurements were carried out at the
central laboratory of
Tikrit Teaching Hospital under the supervision of the quality control system. ELISA kits
were imported from
internationally recognized suppliers, and the sensitivity and specificity of the ELISA Kits
were as per the
standard level of human cytokines.
Radiological Assessment
All patients who underwent baseline brain (and occasionally spinal cord) and 12-month
follow-up MRI. Imaging All
images were acquired on a 1.5 Tesla MR scanner (SIEMENS MAGNETOM Avanto/Siemens Magnetom
Espree) using a
standard multiple sclerosis protocol including axial and sagittal T2-weighted, T2 FLAIR, T1
pre- and
post-gadolinium enhanced sequences, and diffusion-weighted imaging (DWI). MRI disease
activity was measured by
increased T2 (new or enlarging T2 lesions), enhancing, and T1-hypointense (black hole)
lesions. The preoperative
MRI was reviewed by a neuroradiologist who was blinded to clinical entry and laboratory
timelines.
Materials and Equipment
All reagents and materials used in this study were of clinical diagnostic quality. Blood
collection sets,
vacutainers, centrifugation tubes, and pipettes were supplied by the central laboratory of
the hospital.
IL-6, TNF-α, and sNfL ELISA kits were obtained from internationally accredited
manufacturers. MRI
studies were performed on the Siemens 1.5T system of the hospital. Patients were examined
clinically using
standardized neurological examination instruments and EDSS scoring forms.
Statistical Analysis
The data was analyzed with SPSS version 26. Summary statistics (means, standard deviations,
percentages) were
calculated for patient demographics, clinical characteristics, and biomarker levels.
Associations between
peripheral inflammatory markers and clinical/radiological disease activity were investigated
with correlation
analyses (Pearson, when applicable, or Spearman). Multiple linear and logistic regression
models were used to
adjust for key confounding variables, including age, sex, EDSS score, duration of disease,
prior relapses, and
disease-modifying therapy (DMT) usage. To control the potential inflation of type I error
due to multiple
testing, the Benjamini-Hochberg correction method was applied to all p-values derived from
correlation
analyses. Statistical significance was considered with a p-value < 0.05 for all
the analyses.
Results
The discovery cohort included 250 patients who had RRMS. Most participants were female (64 %) with an average age of 34.6 years. The patients had an average disease duration of 6.2 years, and their entry EDSS scores indicated mild to moderate disability levels. The disease-modifying treatment usage rate among patients reached 78 percent. The annualized relapse rate of the sample population reached 1.4 according to Table 1.
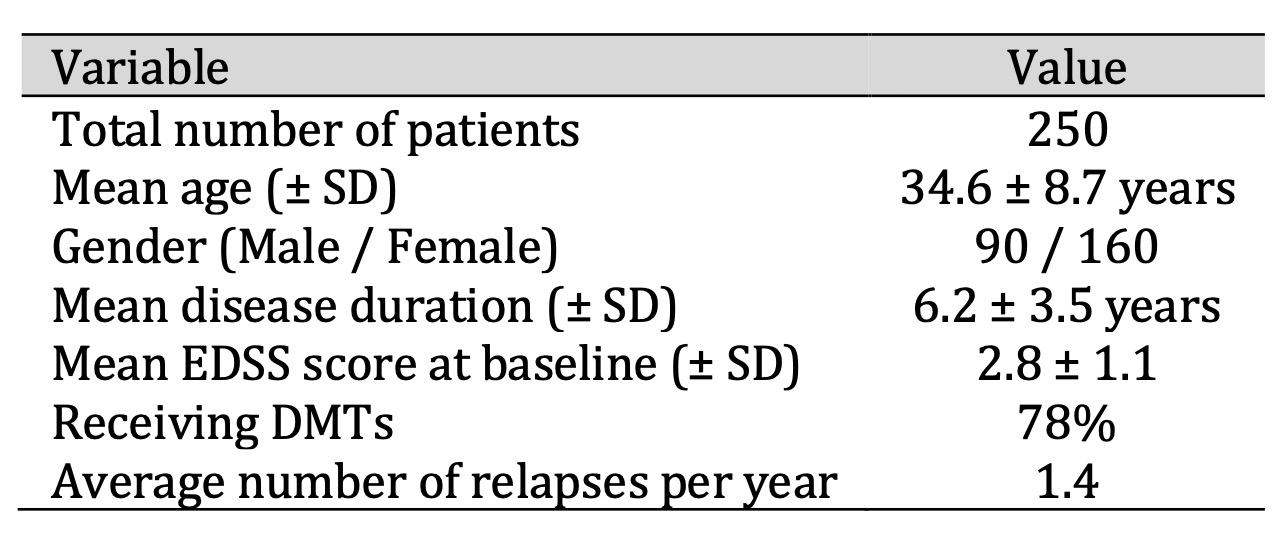
Table 1: Demographic and Clinical Characteristics of the Study Population (n = 250)
As shown in Table 2, elevated inflammatory indices were observed in a considerable number of patients. WBC counts were within normal limits; however, hs-CRP and ESR indicated a low-grade systemic inflammation. The increased IL-6 and TNF-α levels, above the normal reference range, indicated peripheral pro-inflammatory action. The sNfL group average level—a marker of axonal injury—was also significantly raised, indicative of continued neurodegeneration in a higher proportion of patients.
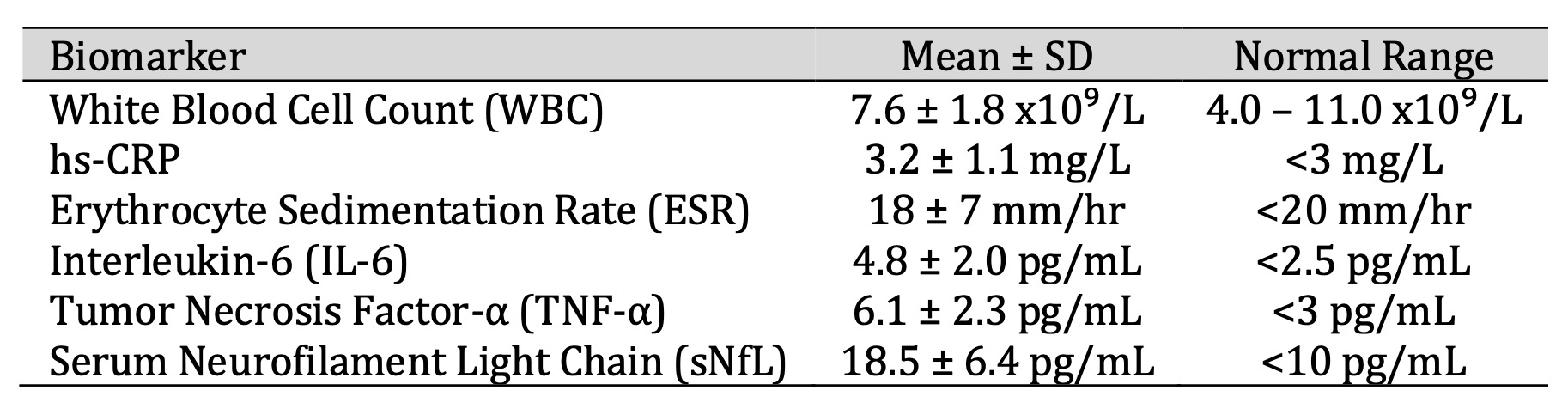
Table 2: Peripheral Inflammatory Biomarkers
On radiologic examination, a reduction of acute inflammatory activity occurred over time with a reduced number of new/enlarging T2 lesions and contrast-enhancing lesions at the 12-month follow-up. Nevertheless, T1 black holes increased slightly, indicating ongoing axonal loss. These data suggest some patients had reduced disease activity (probably because of treatment), but structural damage still accrued in the remainder, as shown in Table 3 and Fig. 1.
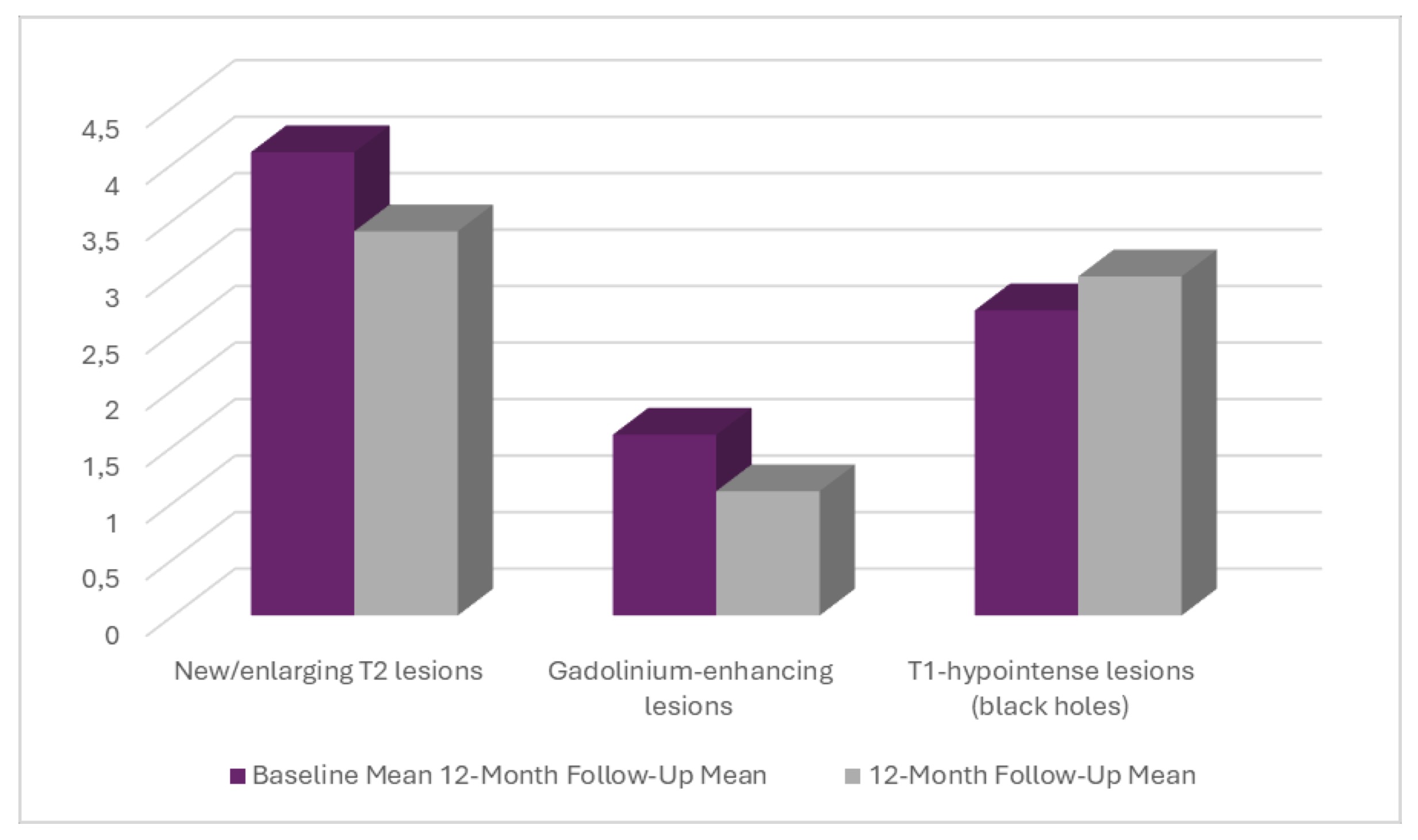
Fig. 1: MRI Findings at Baseline and 12-Month Follow-Up.
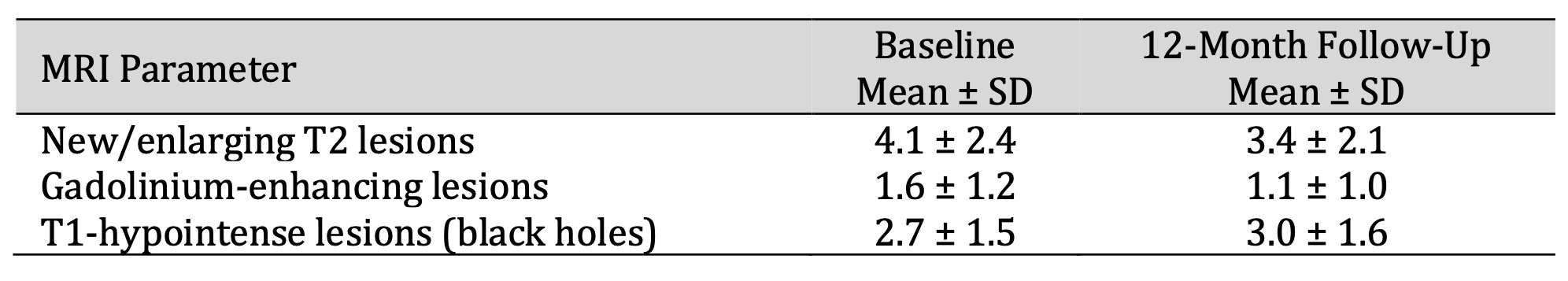
Table 3: MRI Findings at Baseline and 12-Month Follow-Up
Table 4 shows a statistical correlation analysis that revealed significant positive correlations of peripheral inflammatory markers with the clinical and radiological activity of the disease. Association of sNfL with MRI lesion load (r = 0.52, p < 0.001) and EDSS (r = 0.49) were highest, potentially identifying it as the optimal marker for active neurodegeneration. Moderate correlations of IL-6 and TNF-α with disease activity further indicated their suitability as biomarkers for monitoring of RRMS.
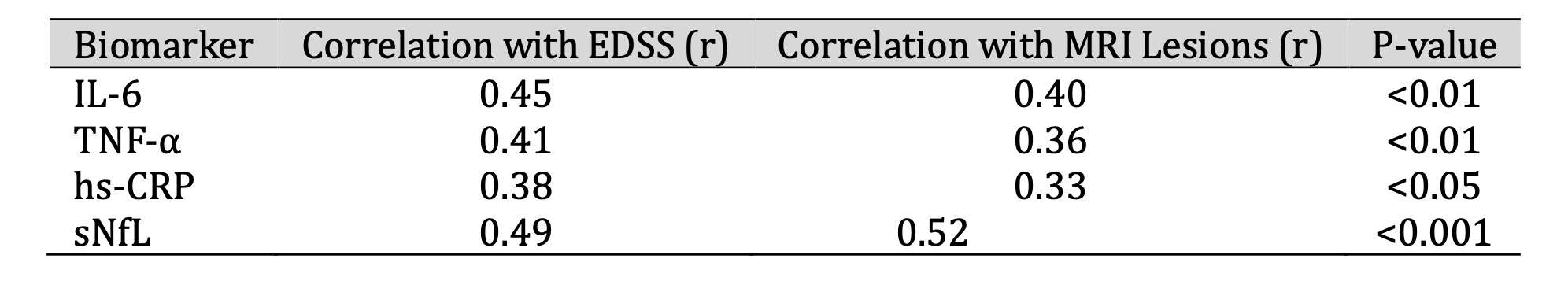
Table 4: Correlation Between Inflammatory Biomarkers and Disease Activity
As demonstrated in Fig. 2, sNfL had the strongest links with both disability (EDSS, r = 0.49, p<0.001***highly significant) and MRI lesion activity (r = 0.52), making it a clear standout among the biomarkers. The other markers—IL-6, TNF-α, (p<0.01**very significant), and hs-CRP (p < 0.05) showed only moderate associations, and these were less convincing once statistical corrections were applied. This suggests that sNfL is a more dependable signal of disease activity and progression in MS than general inflammation markers.
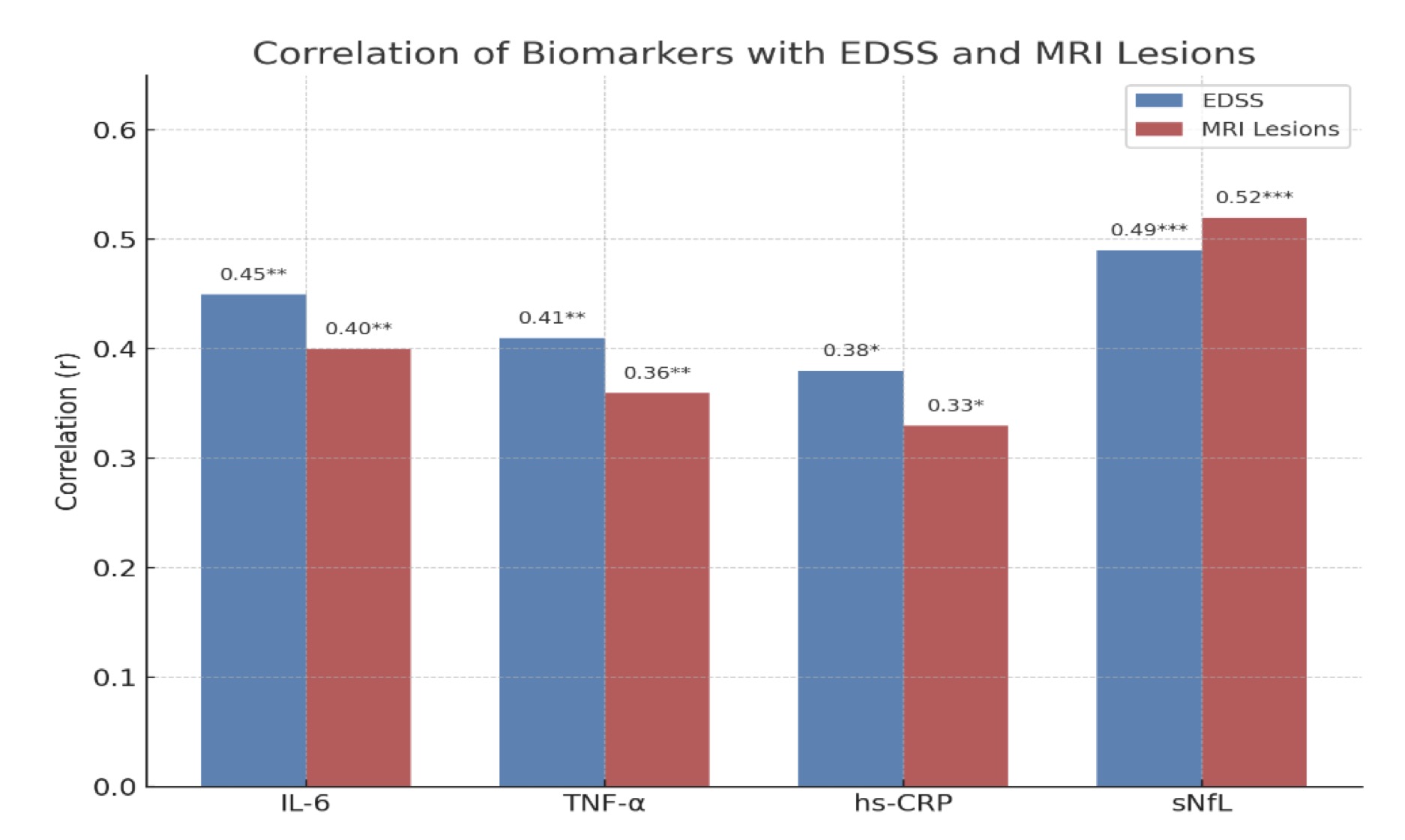
Fig. 2: Correlation of Biomarkers with Disability and MRI Lesion Activity in Multiple Sclerosis.
This network figure clearly shows how different biomarkers relate to both clinical disability (EDSS) and MRI lesion load. Notably, sNfL has the strongest correlations with both MRI lesions (0.52) and EDSS (0.49), reinforcing its role as a sensitive indicator of neuroaxonal damage and disease activity. In contrast, general inflammatory markers like IL-6, TNF-α, and hs-CRP show moderate correlations, suggesting their suitability as biomarkers for monitoring of RRMS. Overall, the network emphasizes that neuroaxonal markers may provide more clinically relevant insights into disease progression in multiple sclerosis, as demonstrated in Fig. 3.
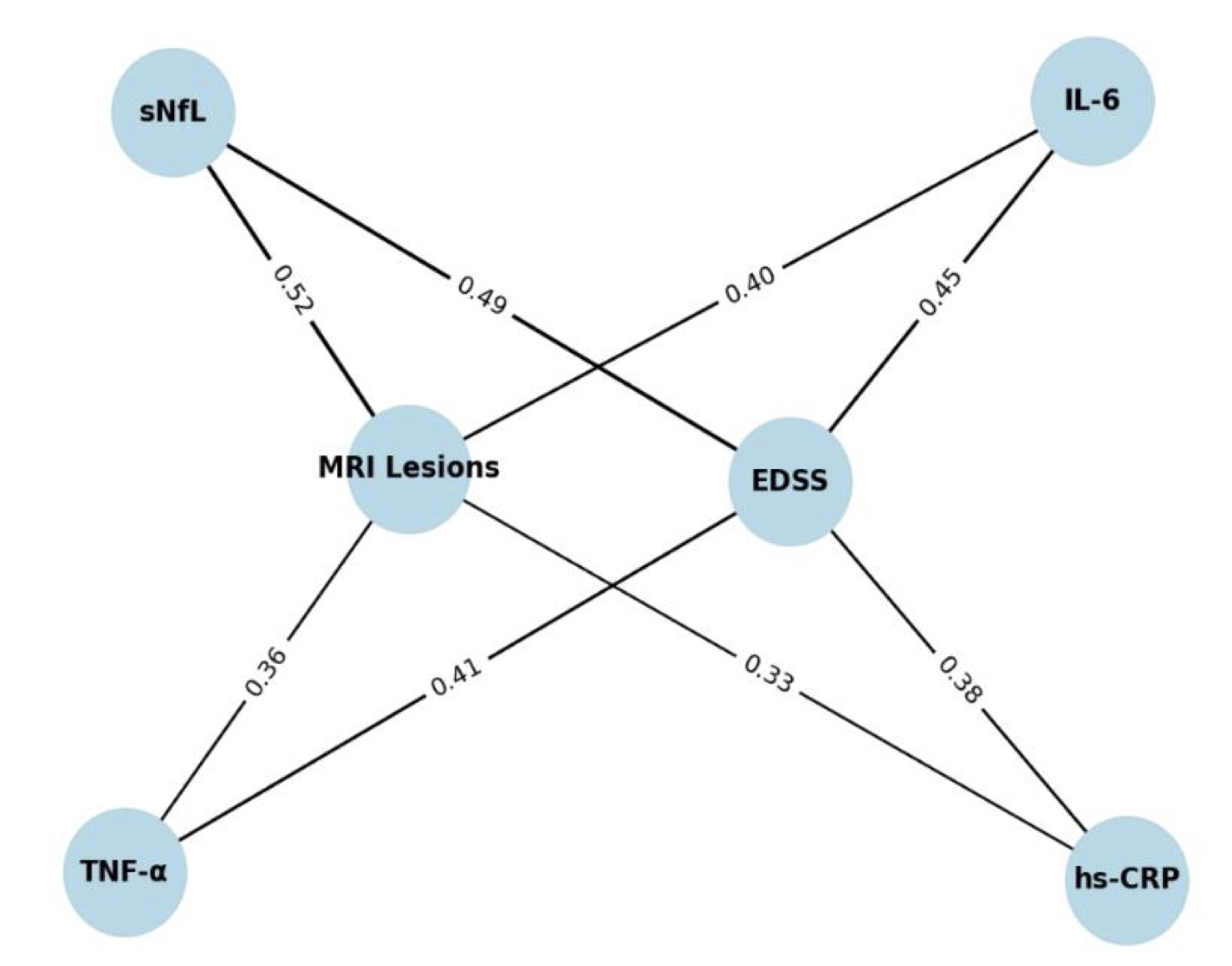
Fig. 3: Network of Correlations Between Biomarkers, Disability (EDSS), and MRI Lesions in Multiple Sclerosis.
Discussion
Regular monitoring of disease activity in relapsing-remitting multiple sclerosis (RRMS) is essential for achieving the best possible clinical outcomes. Biomarkers hold promise in this regard, as they can offer meaningful insights into disease activity when they accurately mirror the underlying pathological processes driving MS (Tatomir et al., 2024).
A distinctive aspect of our study was utilizing data from a hospital-based observational cohort, including private neurology clinics and Tikrit Teaching Hospital in Iraq. Our approach more accurately reflects the diversity and complexity of "real-life" patient care compared to research conducted in controlled laboratory settings. Clinicians routinely encounter a wide range of symptoms and disease progressions, making the findings highly relevant. It was observed that regional factors such as genetics, environment, and healthcare systems significantly impact disease activity, a topic that hasn’t received much attention in multiple sclerosis research. Unlike most prior studies in Western populations, a composite panel of biomarkers—IL-6, TNF-α, hs CRP, ESR, and sNfL—was studied in relation to clinical outcomes, disability scores, and MRI changes in RRMS. This approach adds balance to the existing literature and offers insights that may be valuable both locally and for the broader global understanding of MS. Considering these factors together, rather than in isolation, offers a clearer and more comprehensive view of how disease activity progresses in patients over time.
The appearance of IL-6 and TNF-α early in our cohort occurred sooner than that demonstrated by Paul et al. (2019), suggesting population-specific or disease phenotype heterogeneity. The elevated hs CRP and ESR provide additional evidence for low-grade systemic inflammation, potentially involving the peripheral immune system, as a cause of blood–brain barrier breakdown and CNS demyelination, as depicted by Haschka et al. (2020).
Notably, a positive correlation between sNfL levels and MRI lesion load (r = 0.52; p < 0.001) was found, further supporting its value as a sensitive marker of neuroaxonal injury. This aligns with recent proteomic findings indicating that sNfL can predict long-term outcomes—Tan et al. (2024)—and confirms results from large international cohorts showing the clinical usefulness of sNfL for routine practice and treatment choices (Brune et al., 2022).
Over 12 months, decreases in T2 and gadolinium-enhancing lesion volumes paralleled those of Singer et al. (2023) regarding DMT efficacy. Nevertheless, persistence of T1-hypointense (“black hole”) lesions reflects continual chronic neurodegeneration despite inflammatory containment and is consistent with findings of York et al. (2022), highlighting the value of quantitative MRI in examining lesion pathology.
Our results also add to previous evidence on cytokine-induced activation of macrophages and neuronal health by Muñoz Garcia et al. (2021), and agreed with Kalincik et al. (2023), who proposed combining biomarker profiling following clinical phenotypes to individualize treatment in highly active RRMS.
Our regional Iraqi data strongly suggest that ethnicity and genetic background could affect the progression of MS and agree with Harari et al. (2023), who correlated accelerated disability progression with population-specific neuronal pathway alterations. This emphasizes the essential need for area-specific investigations to decrypt regional disease mechanisms as well as to enhance treatment options.
In agreement with Harper et al. (2024) study, our longitudinal MRI evolution is similar to the trends observed in the MRI aspect of disease in patients with recurrent pancreatitis., that stressed the importance of early structural damage and lesion heterogeneity in MS—both especially relevant in such a young, early MS population prone to experience greater structural rather than inflammatory damage—, underlining the requirement of targeting acute inflammation and chronic neurodegeneration hand in hand in MS management, and the added value of combining inflammation and neuroaxonal biomarker assessment in the real-life MS patient monitoring.
In Tatomir et al. (2024) pilot study, a combined MS disease activity (MSDA) score was developed using six biomarkers—RGC-32, FasL, IL-21, SIRT1, p-SIRT1, and JNK1 p54—to assess relapses and treatment response to glatiramer acetate in RRMS patients. The score showed strong sensitivity, specificity, and correlation with disability levels, though larger trials are still needed for confirmation. When compared with the previous abstract, both studies agree that biomarkers are valuable tools for monitoring MS activity and guiding therapy. However, our study focused on well-established markers of inflammation and neurodegeneration, while Tatomir et al. (2024) proposed a new scoring system based on a different biomarker set, making it a complementary but less validated approach.
Our study and Salah et al.'s (2023) study highlight the value of blood-based biomarkers in understanding RRMS, though they focus on slightly different aspects. The Salah et al.'s (2023) study showed that higher levels of ICAM-1, CRP, NLR, and IgM were linked with greater disability, especially in patients with cerebellar and brainstem involvement, pointing to their role in capturing inflammatory activity. In contrast, our study emphasized IL-6, TNF-α, hs-CRP, ESR, and sNfL, which not only correlated with disability and MRI lesion load but also reflected ongoing neurodegeneration through the persistence of T1 black holes. Together, these findings suggest that while ICAM-1 and related markers may highlight inflammation and disability, IL-6, TNF-α, and sNfL could provide a clearer picture of long-term disease progression.
Olsson et al., (2020) study and our study highlight the growing importance of blood-based biomarkers in understanding and monitoring multiple sclerosis (MS) activity. Olsson et al., (2020) study showed that patients with active MS had significantly higher levels of calprotectin, suggesting it may be a practical marker of innate immune activation, whereas markers of epithelial barrier function were not strongly linked to disease activity. Similarly, our study found that elevated IL-6, TNF-α, hs-CRP, ESR, and serum neurofilament light chain (sNfL) correlated with disability scores and MRI lesion burden in RRMS patients, making them useful indicators of ongoing inflammation and neurodegeneration. Taken together, these findings suggest that while not all biomarkers are equally informative, markers like calprotectin, sNfL, and key cytokines could play an essential role in tracking disease activity and guiding more tailored treatment strategies.
Our findings add to the growing body of evidence that both inflammatory and neurodegenerative processes contribute to disease activity and long-term disability in multiple sclerosis. The observed associations between IL-6, TNF-α, hs-CRP, ESR, and sNfL with clinical disability and MRI lesion burden highlight the clinical utility of peripheral inflammatory biomarkers as accessible indicators of ongoing disease activity. At the same time, research by Ciubotaru et al., (2025) on neurodegenerative markers such as GFAP, CHI3L1, tau, kynurenines, and lipid metabolites underscores their value in capturing progressive axonal and glial damage that may not be fully reflected by inflammatory markers alone. Together, these findings support the integration of both inflammatory and neurodegenerative biomarkers into multi-marker panels, which, when combined with advanced imaging, could improve disease monitoring, aid in early identification of patients at risk of rapid progression, and ultimately support more personalized therapeutic strategies in MS.
Finally, our study is novel and notable for analyzing a comprehensive set of biomarkers rather than focusing only on one or two. By combining MRI results with clinical assessments, this broader approach allowed us to evaluate inflammation, acute-phase responses, and axonal injury simultaneously. This holistic perspective may help in developing more personalized treatment plans and improving disease activity monitoring. Additionally, our findings suggest that monitoring RRMS activity in real-world settings could be improved by integrating imaging, clinical assessments, and peripheral biomarkers. This research marks a significant step toward achieving biomarker-guided care for patients; however, further validation of these results requires additional studies involving larger and more diverse populations.
Conclusion
This study highlights the significance of peripheral inflammatory biomarkers—particularly IL-6, TNF-α, and serum neurofilament light chain—in assessing disease activity in relapsing-remitting multiple sclerosis. Being well correlated with clinical disability and MRI lesion load, they have promising applications as a valid and noninvasive marker for the evaluation of neuroinflammation and neurodegeneration. Although DMTs effectively suppressed the development of acute inflammatory lesions, continuous axonal injury persists, indicating the importance of multi-biomarker assessment in clinical routine. These results open new horizons for individualized management strategies where peripheral markers of inflammation are used for the optimization of personalized treatment and patient prognosis. Future studies need to confirm and extend these findings in ethnically diverse populations to support and improve biomarker-based strategies in MS management.
Acknowledgements
The author acknowledges with gratitude all the support and cooperation received from the Department of Neurology at Tikrit Teaching Hospital during the period of the study. The authors also wish to express their gratitude to the patients involved and to the laboratory and radiology staff for their technical support. The authors would like to acknowledge their supervising physicians and academic mentors, whose supervision was fundamental in accomplishing this study.
Disclosure Statement
The authors have nothing to disclose.
References
| 1 | Boldrini, V. O., Marques, A. M., Quintiliano, R. P. S., et al. (2022). Cytotoxic
B cells in relapsing-remitting multiple sclerosis patients. Frontiers in
Immunology, 13, 750660. https://doi.org/10.3389/fimmu.2022.750660
https://doi.org/10.3389/fimmu.2022.750660 |
| 2 | Boscolo Galazzo, I., Brusini, L., Akinci, M., et al. (2022). Unravelling the
MRI‐based microstructural signatures behind primary progressive and
relapsing-remitting multiple sclerosis phenotypes. Journal of Magnetic Resonance
Imaging, 55(1), 154-163. https://doi.org/10.1002/jmri.27806
https://doi.org/10.1002/jmri.27806 |
| 3 | Brune, S., Høgestøl, E. A., de Rodez Benavent, S. A., Berg-Hansen, P., Beyer, M.
K., Leikfoss, I. S., Bos, S. D., Sowa, P., Brunborg, C., Andorra, M., Pulido
Valdeolivas, I., Asseyer, S., Brandt, A., Chien, C., Scheel, M., Blennow, K.,
Zetterberg, H., Kerlero de Rosbo, N., Paul, F., ... Harbo, H. F. (2022). Serum
neurofilament light chain concentration predicts disease worsening in multiple
sclerosis. Multiple Sclerosis Journal, 28(12), 1859-1870.
https://doi.org/10.1177/13524585221097296
https://doi.org/10.1177/13524585221097296 |
| 4 | Campbell, J. A., Henson, G. J., Ngwa, V. F., Ahmad, H., Taylor, B. V., van der
Mei, I., MSBase Australian Researchers, & Palmer, A. J. (2025). Estimation of
transition probabilities from a large cohort (> 6000) of Australians living with
multiple sclerosis (MS) for changing disability severity classifications, MS
phenotype, and disease-modifying therapy classifications. Pharmacoeconomics,
43(2), 223-239. https://doi.org/10.1007/s40273-024-01417-4
https://doi.org/10.1007/s40273-024-01417-4 |
| 5 | Ciubotaru, A., Smihor, M. I., Grosu, C., Alexa, D., Covali, R., Anicăi, R.-C.,
Păvăleanu, I., Cucu, A. I., Bobu, A. M., Ghiciuc, C. M., & Ignat, E. B. (2025).
Neurodegenerative Biomarkers in Multiple Sclerosis: At the Interface Between
Research and Clinical Practice. Diagnostics, 15(9), 1178.
https://doi.org/10.3390/diagnostics15091178
https://doi.org/10.3390/diagnostics15091178 |
| 6 | Etta, I., Elballushi, R., Kolesnyk, V., et al. (2023). Comparison of
pharmacological therapies in relapse rates in patients with relapsing-remitting
multiple sclerosis. Cureus, 15(9), e45454. https://doi.org/10.7759/cureus.45454
https://doi.org/10.7759/cureus.45454 |
| 7 | Harari, G., Gurevich, M., Dolev, M., et al. (2023). Faster progression to
multiple sclerosis disability is linked to neuronal pathways associated with
neurodegeneration: An ethnicity study. PLoS One, 18(2), e0280515.
https://doi.org/10.1371/journal.pone.0280515
https://doi.org/10.1371/journal.pone.0280515 |
| 8 | Harper, J. G., York, E. N., Meijboom, R., et al. (2024). Quantitative T1 brain
mapping in early relapsing-remitting multiple sclerosis: Longitudinal changes,
lesion heterogeneity and disability. European Radiology, 34(6), 3826-3839.
https://doi.org/10.1007/s00330-023-10351-6
https://doi.org/10.1007/s00330-023-10351-6 |
| 9 | Haschka, D., Tymoszuk, P., Bsteh, G., Petzer, V., Berek, K., Theurl, I., Berger,
T., & Weiss, G. (2020). Expansion of neutrophils and classical and nonclassical
monocytes as a hallmark in relapsing-remitting multiple sclerosis. Frontiers in
Immunology, 11, 594. https://doi.org/10.3389/fimmu.2020.00594
https://doi.org/10.3389/fimmu.2020.00594 |
| 10 | Heesen, C., Röver, C., Salem, S., et al. (2024). Treatment effect modifiers of
immunotherapies for relapsing-remitting multiple sclerosis-A systematic review
and meta-analysis. Multiple Sclerosis Journal - Experimental, Translational and
Clinical, 10(4), 20552173241274618. https://doi.org/10.1177/20552173241274618
https://doi.org/10.1177/20552173241274618 |
| 11 | Holtzer, R., Foley, F. W., Motl, R. W., et al. (2024). Brain hemodynamic
responses and fall prediction in older adults with multiple sclerosis. Multiple
Sclerosis, 30(13), 1664-1673. https://doi.org/10.1177/13524585241277400
https://doi.org/10.1177/13524585241277400 |
| 12 | Kalincik, T., Sharmin, S., Roos, I., et al. (2023). Comparative effectiveness of
autologous hematopoietic stem cell transplant vs fingolimod, natalizumab, and
ocrelizumab in highly active relapsing-remitting multiple sclerosis. JAMA
Neurology, 80(7), 702-713. https://doi.org/10.1001/jamaneurol.2023.1184
https://doi.org/10.1001/jamaneurol.2023.1184 |
| 13 | Muñoz-Garcia, J., Cochonneau, D., Télétchéa, S., Moranton, E., Lanoe, D., Brion,
R., Lézot, F., Heymann, M.-F., & Heymann, D. (2021). The twin cytokines
interleukin-34 and CSF-1: Masterful conductors of macrophage homeostasis.
Theranostics, 11(4), 1568-1593. https://doi.org/10.7150/thno.50683
https://doi.org/10.7150/thno.50683 |
| 14 | Olsson, A., Gustavsen, S., Chenoufi Hasselbalch, I., Langkilde, A. R.,
Sellebjerg, F., Oturai, A. B., & Søndergaard, H. B. (2020). Biomarkers of
inflammation and epithelial barrier function in multiple sclerosis. Multiple
Sclerosis and Related Disorders, 46, 102520.
https://doi.org/10.1016/j.msard.2020.102520
https://doi.org/10.1016/j.msard.2020.102520 |
| 15 | Paul, A., Comabella, M., & Gandhi, R. (2019). Biomarkers in multiple sclerosis.
Cold Spring Harbour Perspectives in Medicine, 9(3), a029058.
https://doi.org/10.1101/cshperspect.a029058
https://doi.org/10.1101/cshperspect.a029058 |
| 16 | Salah Abdal-Aziz, H., Abd El-Wahed, M. R., El-Said, H. H., & Mohamed, W. S.
(2023). The association of inflammatory markers and ICAM-1 with neurological
disabilities in relapsing-remitting multiple sclerosis (RRMS) patients. Zagazig
University Medical Journal, 29(1, Suppl.), 80-89.
https://doi.org/10.21608/zumj.2020.36548.1901
https://doi.org/10.21608/zumj.2020.36548.1901 |
| 17 | Singer, B. A., Arnold, D. L., Drulovic, et al. (2023). Diroximel fumarate in
patients with relapsing-remitting multiple sclerosis: Final safety and efficacy
results from the phase 3 EVOLVE-MS-1 study. Multiple Sclerosis Journal, 29(14),
1795-1807. https://doi.org/10.1177/13524585231205708
https://doi.org/10.1177/13524585231205708 |
| 18 | Swanberg, K. M., Kurada, A. V., Prinsen, H., et al. (2022). Multiple sclerosis
diagnosis and phenotype identification by multivariate classification of in vivo
frontal cortex metabolite profiles. Scientific Reports, 12, 13888.
https://doi.org/10.1038/s41598-022-17741-8
https://doi.org/10.1038/s41598-022-17741-8 |
| 19 | Tan, I. L., Modderman, R., Stachurska, A., Almeida, R., de Vries, R., Heersema,
D. J., Gacesa, R., Wijmenga, C., Jonkers, I. H., Meilof, J. F., & Withoff, S.
(2024). Potential biomarkers for multiple sclerosis stage from targeted
proteomics and microRNA sequencing. Brain Communications, 6(4), fcae209.
https://doi.org/10.1093/braincomms/fcae209
https://doi.org/10.1093/braincomms/fcae209 |
| 20 | Tatomir, A., Anselmo, F., Boodhoo, D., Chen, H., Mekala, A. P., Nguyen, V.,
Cuevas, J., Rus, V., & Rus, H. (2024). Multiple sclerosis disease activity, a
multi-biomarker score of disease activity and response to treatment in multiple
sclerosis. Frontiers in Immunology, 15, 1338585.
https://doi.org/10.3389/fimmu.2024.1338585
https://doi.org/10.3389/fimmu.2024.1338585 |
| 21 | Weinstock-Guttman, B., Bermel, R., Cutter, G., et al. (2022). Ocrelizumab
treatment for relapsing-remitting multiple sclerosis after a suboptimal response
to previous disease-modifying therapy: A nonrandomized controlled trial.
Multiple Sclerosis, 28(5), 790-800. https://doi.org/10.1177/13524585211035740
https://doi.org/10.1177/13524585211035740 |
| 22 | Wiyani, A., Badgujar, L., Khurana, V., et al. (2021). How have economic
evaluations in relapsing multiple sclerosis evolved over time? A systematic
literature review. Neurology and Therapy, 10(2), 557-583.
https://doi.org/10.1007/s40120-021-00264-1
https://doi.org/10.1007/s40120-021-00264-1 |
| 23 | Wurtz, L. I., Knyazhanskaya, E., Sohaei, D., et al. (2024). Identification of
brain-enriched proteins in CSF as biomarkers of relapsing-remitting multiple
sclerosis. Clinical Proteomics, 21, 42.
https://doi.org/10.1186/s12014-024-09494-5
https://doi.org/10.1186/s12014-024-09494-5 |
| 24 | York, E. N., Thrippleton, M. J., Meijboom, R., Hunt, D. P. J., & Waldman, A. D.
(2022). Quantitative magnetisation transfer imaging in relapsing-remitting
multiple sclerosis: A systematic review and meta-analysis. Brain Communications,
4(2), fcac088. https://doi.org/10.1093/braincomms/fcac088
https://doi.org/10.1093/braincomms/fcac088 |
| 25 | Ziemssen, T., Bass, A. D., Van Wijmeersch, B., et al. (2025). Long-term efficacy
and safety of alemtuzumab in participants with highly active MS: TOPAZ clinical
trial and interim analysis of TREAT-MS real-world study. Therapeutic Advances in
Neurological Disorders, 18, 17562864241306575.
https://doi.org/10.1177/17562864241306575
https://doi.org/10.1177/17562864241306575 |
| 26 | Zivadinov, R., Bergsland, N., Jakimovski, D., et al. (2024). Thalamic atrophy
and dysconnectivity are associated with cognitive impairment in a multi-centre,
clinical routine, real-world study of people with relapsing-remitting multiple
sclerosis. NeuroImage: Clinical, 42, 103609.
https://doi.org/10.1016/j.nicl.2024.103609
https://doi.org/10.1016/j.nicl.2024.103609 |